sampling , sample preparation , analysis , interpretation , preparation of report and pipette , burette , chemical balance and least count ( for B.A. , B.Com. and B.Sc. ) analytical chemistry unit 3 and 4
- Steps in chemical analysis -
You've outlined the key steps in chemical analysis correctly. Here's a bit more detail on each of those steps:
You've outlined the key steps in chemical analysis correctly. Here's a bit more detail on each of those steps:
1. Sampling: - This is the process of selecting a representative portion of the material or substance to be analyzed. The accuracy of the analysis depends on the quality of the sample, so it's essential to collect it carefully.
2. Sample Preparation:- Once the sample is collected, it often needs to be prepared. This could involve grinding, filtering, diluting, or any other steps necessary to make it suitable for the specific analysis method.
3. Analysis:- In this step, you perform the actual chemical analysis. This can involve various techniques, such as spectroscopy, chromatography, titration, or any other method relevant to the specific analysis goals.
4. Interpretation:- After obtaining the analysis results, you interpret the data in the context of your research or objectives. This step may involve identifying compounds, assessing concentrations, and drawing conclusions based on the data.
5. Preparation of Report:- Once the analysis is complete and interpreted, the findings are documented in a report. This report includes details about the sample, the analysis method, the results, and the conclusions drawn from the analysis.
These steps are fundamental to any chemical analysis, and they are crucial to ensure the accuracy and reliability of the results. Additionally, there may be quality control and validation steps incorporated throughout the process to maintain the integrity of the analysis.
Unit - 4
Use of measuring equipments
# Pipette
A pipette is a laboratory instrument used for accurately measuring and transferring small volumes of liquid. Its primary functions include:
1. Dispensing:- Pipettes are used to draw up a specific volume of liquid from a source container and then dispense that liquid into a target container with precision. This is crucial for experiments and tests that require exact measurements.
2. Mixing:- Pipettes can be used for gentle mixing or homogenizing of liquids, especially when small volumes need to be combined.
3. Dilution:- Pipettes are essential for creating dilutions of solutions by accurately transferring a known volume of a concentrated solution into a new container and adding a specific volume of solvent.
4. Sample preparation:- Pipettes are used in various scientific fields, including biology, chemistry, and clinical diagnostics, for preparing samples for analysis, such as DNA extraction, protein assays, and more.
5. Maintaining sterility:- In microbiology and cell culture work, pipettes with sterile tips are used to ensure that samples remain uncontaminated.
6. Precise measurement:- Pipettes come in various types, such as micropipettes and serological pipettes, each with different measurement ranges. They allow scientists to measure and transfer liquids in the microliter to milliliter range accurately.
Overall, pipettes are fundamental tools in scientific research and clinical laboratories, contributing to accurate and reproducible experiments and analyses.
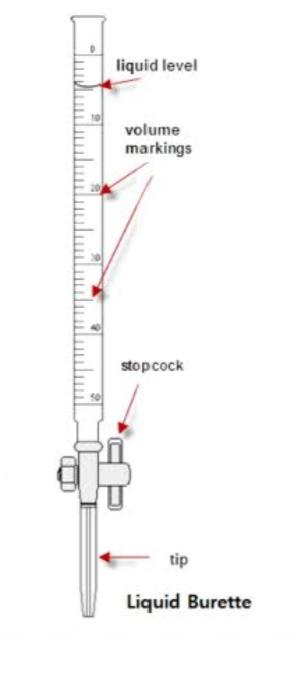
# Burette -
A burette is a laboratory glassware used for precisely measuring and dispensing known volumes of liquid. It consists of a long, graduated tube with a stopcock at the bottom. Burettes are primarily used in titration experiments, where the precise volume of one solution is added to another solution until a chemical reaction reaches its endpoint. Key features and functions of a burette include:
1. Graduations: Burettes are marked with graduated lines along their length, allowing for accurate measurement of the volume of liquid being dispensed. Typically, burettes are graduated to read in milliliters (mL) with subdivisions for even more precise measurements.
2. Stopcock: The stopcock at the bottom of the burette can be opened or closed to control the flow of liquid. This precise control is essential during titration, where small increments of liquid are added until the reaction is complete.
3. Titration: Burettes are commonly used in acid-base titrations, where a known volume of one solution (titrant) is gradually added to another solution (analyte) until a chemical reaction reaches its endpoint, often indicated by a color change or other observable change.
4. Accuracy: Burettes are known for their accuracy in measuring and dispensing liquids. They are designed to minimize parallax error, where the observer's eye is not at the same level as the liquid's surface, by allowing the liquid level to align with the graduation .
5. Calibration: Burettes need to be regularly calibrated to ensure their accuracy. Calibration involves checking and adjusting the volume readings to maintain precision.
Overall, burettes are essential tools in quantitative chemical analysis, allowing chemists and scientists to perform titrations with a high degree of precision and accuracy.
# Chemical balance -
In analytical chemistry, a chemical balance, also known as an analytical balance or precision balance, is a highly sensitive measuring instrument used to determine the mass of substances with a very high degree of precision and accuracy. These balances are essential tools for quantitative chemical analysis and research. Here's how they function as a measuring equipment in analytical chemistry:
1. Mass Measurement: The primary purpose of a chemical balance is to measure the mass of substances, whether they are solid, liquid, or powder form. This is crucial for various analytical procedures where knowing the exact mass of a sample is essential.
2. Precision and Accuracy: Chemical balances are designed to provide highly precise and accurate measurements. They can measure to within a fraction of a milligram or even micrograms, depending on the specific balance's capabilities.
3. Sensitivity to Environmental Factors: Analytical balances are extremely sensitive to environmental conditions like air currents and temperature fluctuations. To minimize these influences, they are often placed inside a controlled environment or a draft shield.
4. Calibration: Regular calibration is necessary to maintain the accuracy of a chemical balance. This involves adjusting the balance with standard weights to ensure that it provides accurate measurements.
5. Taring: A balance can be "tared" to zero by adjusting its display when an empty container or vessel is placed on the balance pan. This allows for the accurate measurement of the substance's mass within the container.
6. Analytical Techniques: Chemical balances play a crucial role in various analytical techniques, including gravimetric analysis, where the mass of a substance is used to determine its concentration, and in preparing solutions with precise concentrations.
7. Data Recording: Modern chemical balances often have digital displays and the ability to connect to computers or printers, facilitating data recording and documentation of measurements for analytical reports.
In summary, chemical balances are indispensable measuring instruments in analytical chemistry, enabling scientists to perform quantitative analyses, quality control, and research by accurately determining the mass of substances with a high level of precision and reliability.
# least count -
The term "least count" in analytical chemistry refers to the smallest increment by which a measuring instrument can display or read a value. It represents the level of precision or resolution of the instrument and is typically expressed in the units of measurement being used. The least count is crucial when performing quantitative analyses and measurements in analytical chemistry.
For example, in a laboratory balance used for weighing substances, the least count would be the smallest mass that the balance can accurately measure and display. If the least count of a balance is 0.1 milligram (0.0001 grams), it means the balance can accurately measure mass to the nearest 0.1 milligram.
Similarly, in a graduated cylinder used for volume measurements, the least count would be the smallest volume increment that can be accurately read from the graduated markings. If the least count of a graduated cylinder is 1 milliliter, it means that you can estimate volume measurements to the nearest milliliter.
In analytical chemistry, precision is vital, and understanding the least count of the measuring instruments is essential for obtaining accurate and reliable results. It helps in ensuring that measurements are made with the appropriate level of precision and can impact the precision and accuracy of quantitative analyses.
Done by Rohit Joshi ....
You can comment down your doubts -
Also read this -







Comments
Post a Comment